How is Green Hydrogen produced and used
ENERGY
7 SEPTEMBER 2025
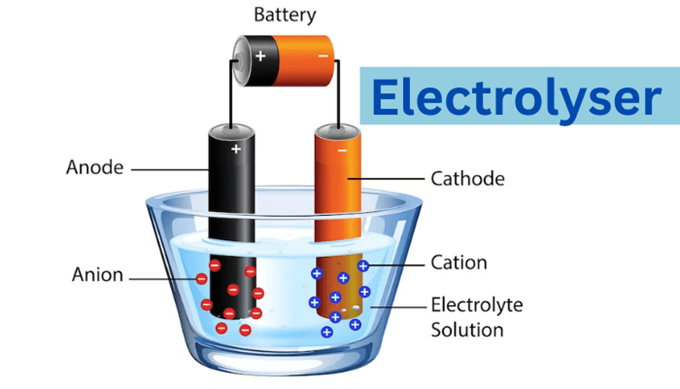
Electrolyser
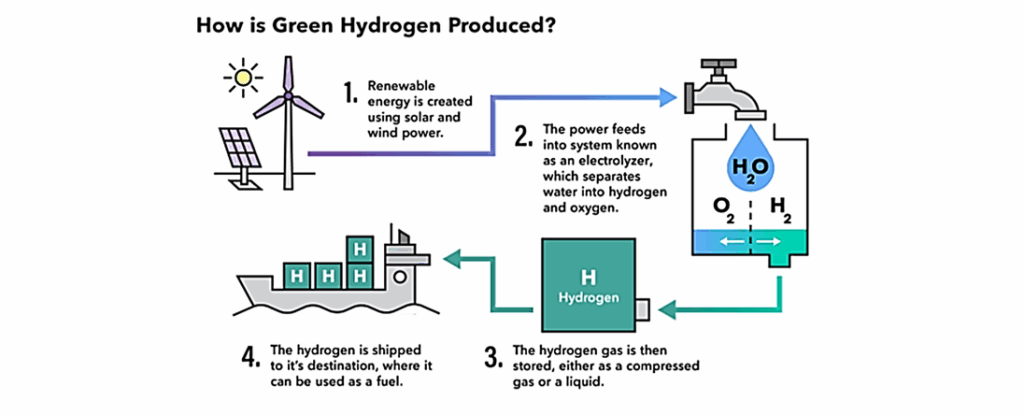
- Renewable energy is created using solar and wind power.
- This power feeds into a system known as an electrolyser.
- An electrolyser splits a water molecule into oxygen, protons, and electrons.
- In an electrochemical reaction at the negative electrode (called the anode), molecular oxygen is released, and the electrons liberated are conducted to the cathode via an external circuit.
- The polymer electrolyte membrane between the cathode and the anode is selective and only allows protons to pass through to the cathode, where they unite with the electrons to form hydrogen molecules.
- These rise as a gas and are collected, compressed, and stored.
- The membrane, typically a fluoropolymer such as NaFion (related to Teflon) is an excellent insulator, and electrons will not pass.
- The hydrogen and oxygen formed are clearly separated.
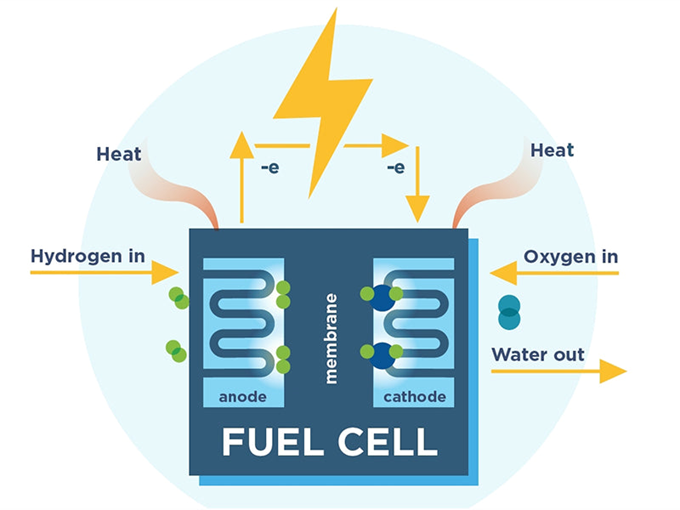
Fuel Cell
- In the locomotive, as in a hydrogen-powered automobile, the above reaction is reversed in the hydrogen fuel cell.
- Hydrogen is brought to the anode, where each molecule is catalytically split into two protons and two electrons.
- The catalysis steps require expensive materials such as platinum, iridium, etc.
- Ongoing research is aimed at replacing these with inexpensive nickel, cobalt, or even iron.
- The protons pass through the membrane to the cathode, where they meet oxygen in air and the electrons that are brought through an external circuit from the anode. Water is formed.
- The electrons flowing through the external circuit constitute the electric current that powers the locomotive.
- The key difference is that a fuel cell converts chemical energy into electricity (e.g., hydrogen and oxygen into water and power), while an electrolyser uses electricity to drive a non-spontaneous reaction to produce chemicals (e.g., water and electricity into hydrogen and oxygen).