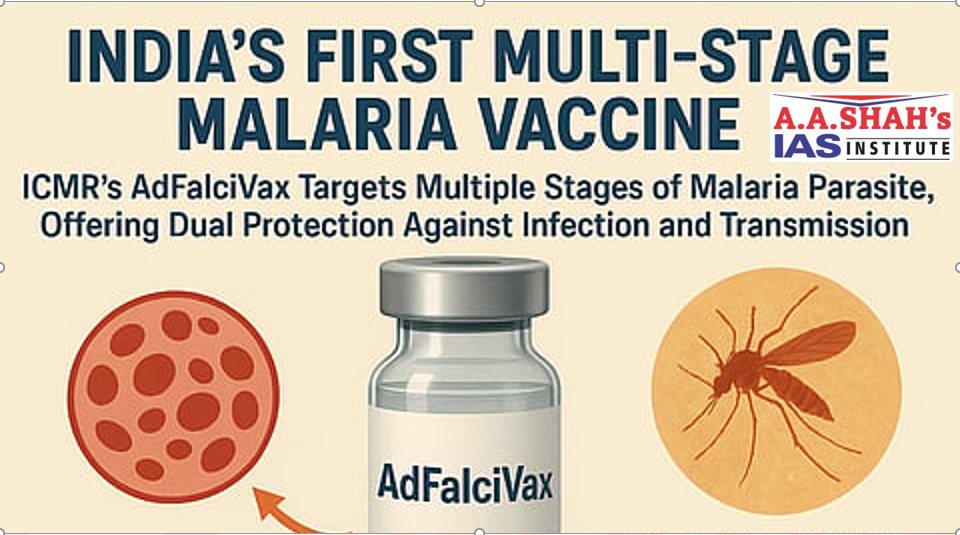
AdFalciVax : India’s first indigenous malaria vaccine
S&T – HEALTH
10 SEPTEMBER 2025
- The Union government has given licences to five firms for manufacturing and commercialisation of its first indigenous multi-stage malaria vaccine developed by the Indian Council of Medical Research (ICMR) and its partners.
- The council said it was an affordable, stable, and scalable solution. It remains effective for more than nine months at room temperature, it said.
- The ICMR has initiated transfer of technology for commercialisation of “a recombinant chimeric multi-stage malaria vaccine (AdFalciVax) against Plasmodium falciparum useful in preventing infection in humans and minimising community transmission”.
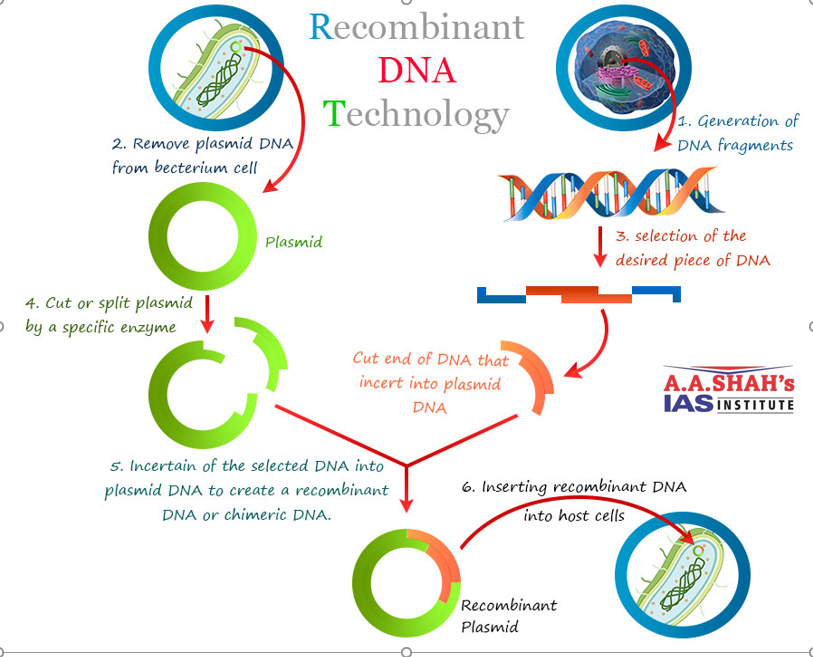
- A chimeric vaccine combines pieces (antigens) from different stages or proteins of the malaria parasite into a single vaccine antigen.
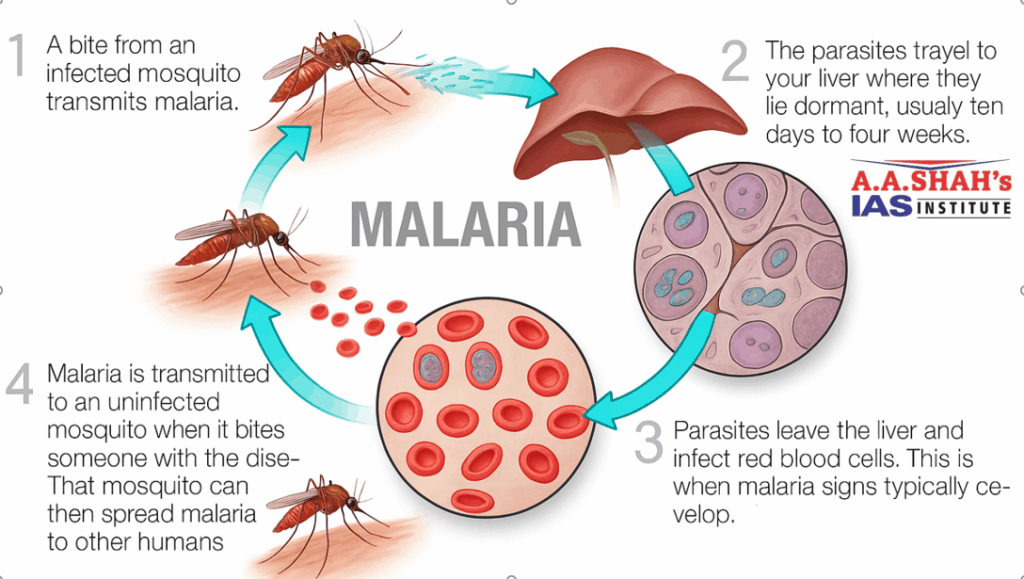
- The idea is to trigger immune responses at multiple points in the parasite’s lifecycle (e.g. liver stage, blood stage, transmission stage).
- They use molecular biology / recombinant DNA techniques to fuse together these antigenic fragments.
- India carries 1.4% of the global malaria case burden, and accounted for 66% of cases in the Southeast Asia region.