Drug makers must comply with revised ‘Schedule M’ norms: govt.
SOCIAL – HEALTH
6 OCTOBER 2025
- The Union Health Ministry has sought strict compliance by drug manufacturers with the revised Schedule M norms for pharmaceutical products in India.
- Licences of non-compliant units would be cancelled, it has warned.
- The direction comes after an emergency meeting with officials of the States and Union Territories, following a report by the Tamil Nadu Drugs Control Department, which found above permissible levels of diethylene glycol (DEG) in samples of Coldrif, a cough syrup brand.
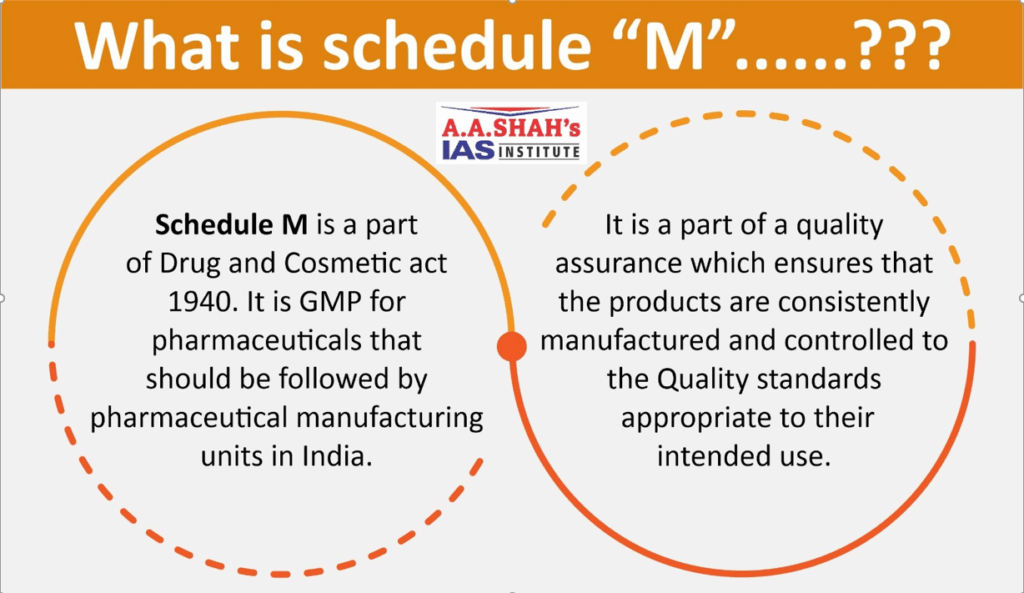
- The revised Schedule M is an updated set of Good Manufacturing Practices (GMP) and regulations for pharmaceutical products in India, part of Drugs and Cosmetics Act, 1940.
- It mandates enhanced quality systems, including the Pharmaceutical Quality System and Quality Risk Management, with a compliance deadline of December 31, 2025.
- The revisions align Indian standards with international GMP guidelines, emphasising product quality and safety, and require new infrastructure, including computerised storage systems and equipment validation.
- Testing of cough syrup brands was initiated following the death of more than 10 children in Rajasthan and Madhya Pradesh.